South Korean chipmaker bats US FDA approval of its non-invasive glucose monitoring device
페이지 정보
작성일23-03-16 11:18관련링크
본문
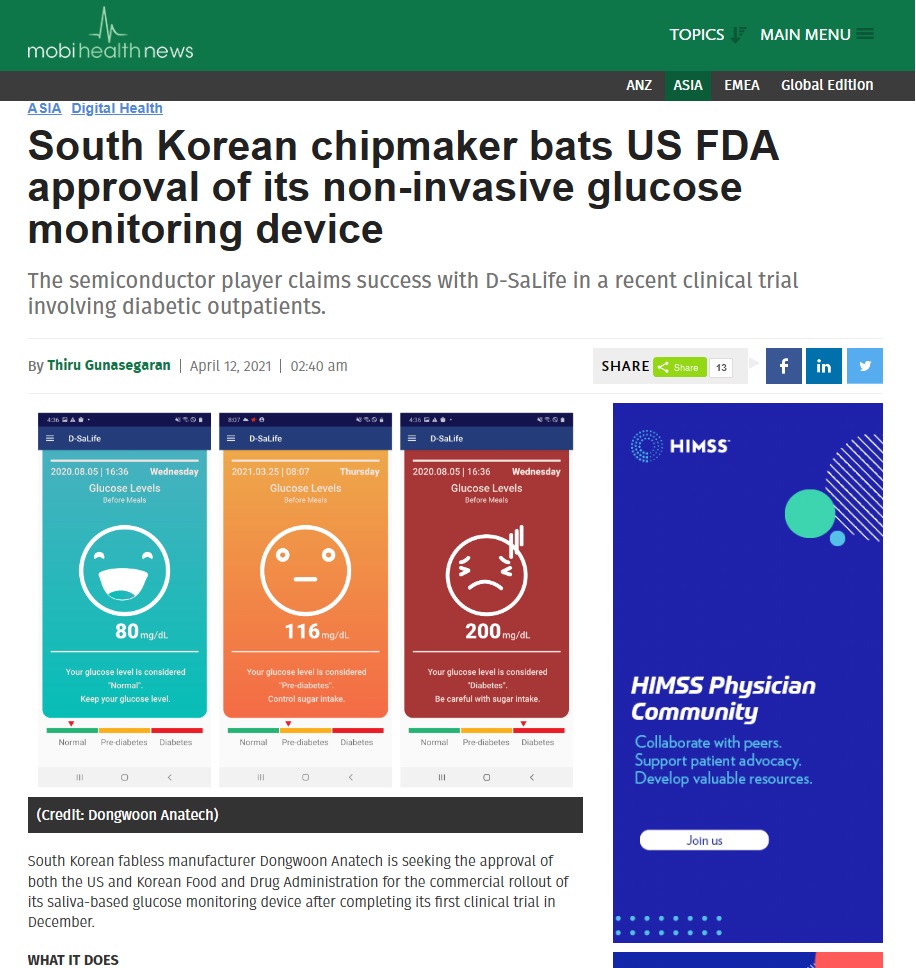
South Korean chipmaker bats US FDA approval of its non-invasive glucose monitoring device The semiconductor player claims success with D-SaLife in a recent clinical trial involving diabetic outpatients. By Thiru Gunasegaran April 12, 2021 02:40 am SHARE 13 (Credit: Dongwoon Anatech) South Korean fabless manufacturer Dongwoon Anatech is seeking the approval of both the US and Korean Food and Drug Administration for the commercial rollout of its saliva-based glucose monitoring device after completing its first clinical trial in December. WHAT IT DOES The glucose monitoring device called D-SaLife, a product of six years of research and development, makes use of microcurrent control technology in determining the glucose level present in a person’s saliva sample. The meter colour codes the result of the reading which is also recorded through a mobile app. WHY IT MATTERS Dongwoon conducted its first exploratory clinical trial in Nowon Eulji Medical Center – a university hospital in Seoul – involving 114 diabetic outpatients. The KOSDAQ-listed chipmaker is also planning to conduct D-SaLife’s clinical trials in the US, though it has not disclosed a date for it. The company has already secured patent recognition for the technology behind D-SaLife in South Korea, Japan and China; more patent applications are pending in Europe, India and the US. MARKET SNAPSHOT D-SaLife is not the only saliva-based glucose test mechanism present today. In 2019, Australian biotech company iQ Global Group introduced its Saliva Glucose Biosensor, a small disposable strip that when exposed to saliva will transmit a sugar level measurement to a nearby smartphone. Non-prick, wearable continuous glucose monitors are also being marketed around the world, like Abbott’s FreeStyle Libre 2 and Symphony by Echo Therapeutics, an early player in this space. Health tech startup Movano is also coming up with a radio frequency-powered CGM. Moreover, there are also minimally invasive ones such as the Dexcom G6 and Biolinq’s upcoming CGM that uses a biosensor. By 2025, the global digital diabetes management market is estimated to reach $17.09 billion at a CAGR of 23%. In 2019, about one in 11 adults suffered from diabetes or about 463 million people worldwide, data from the International Diabetes Federation has shown. ON THE RECORD "Dongwoon Anatech challenges the healthcare market with semiconductor innovation technology and know-how that [we have] accumulated for more than 20 years as a key player of the semiconductor industry," Dongwoon CEO Kim Dongcheol said. "We will become a leader [in diagnostics], focusing on improving the lives of patients and their families through D-SaLife," he added. Tags: Dongwoon Anatech, D-SaLife, Diabetes, innovation, blood glucose monitoring
https://www.mobihealthnews.com/news/asia/south-korean-chipmaker-bats-us-fda-approval-its-non-invasive-glucose-monitoring-device